Nā huahana
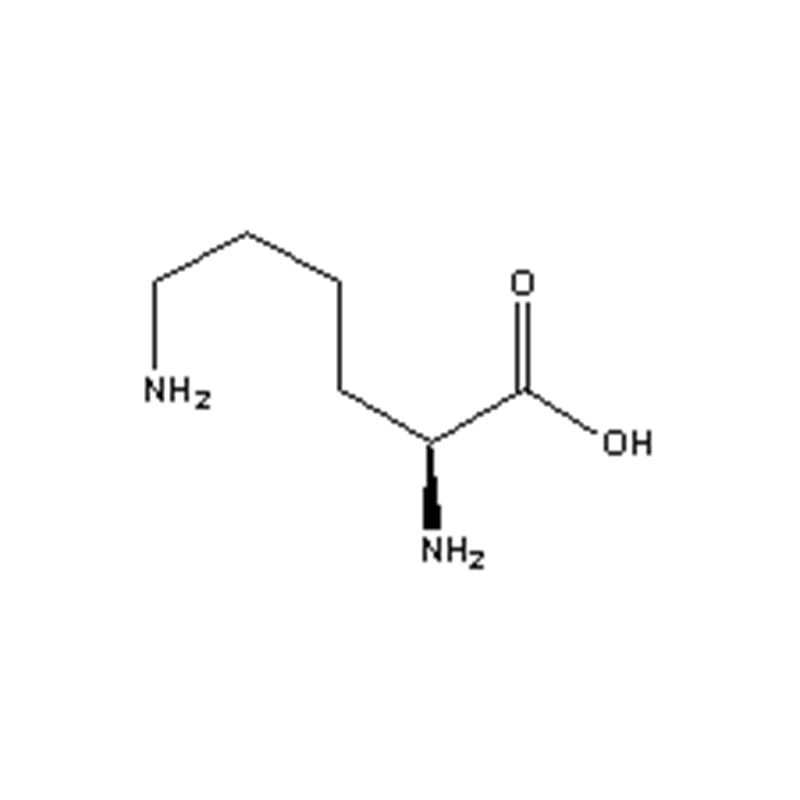
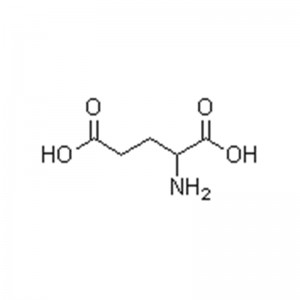
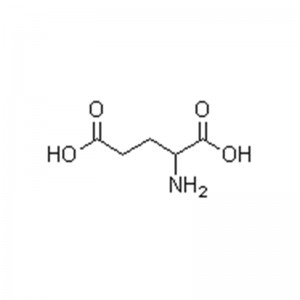
L-lysine
wehewehe
No ka ʻoihana meaʻai.ʻO Lysine kahi mea nui o ka protein.ʻO ia kekahi o nā waikawa amino ʻewalu i hiki ʻole i ke kino o ke kanaka ke synthesize iā ia iho, akā pono loa ia.He mea paʻa meaʻai maikaʻi loa ia.Ma muli o ka nele o ka lysine i ka meaʻai, ua kapa ʻia ʻo ia ʻo "ka amino acid mua".ʻO ka hoʻohui ʻana i ka lysine i nā mea inu, ka laiki, ka palaoa, nā kini a me nā meaʻai ʻē aʻe e hiki ke hoʻomaikaʻi i ka nui o ka hoʻohana ʻana o ka protein, i mea e hoʻoikaika nui ai i ka meaʻai o ka meaʻai, hoʻoikaika i ka ulu a me ka ulu ʻana, hoʻonui i ka makemake, hoʻemi i ka maʻi a hoʻomaikaʻi i ke kino.Hiki ke hoʻohana no ka deodorizing a me ka mālama hou i loko o nā kini.
No ka ʻoihana lāʻau lapaʻau.Hiki ke hoʻohana ʻia ʻo Lysine e hoʻomākaukau i ka infusion amino acid, ʻoi aku ka maikaʻi o ka hopena a me ka liʻiliʻi o nā hopena ʻaoʻao ma mua o ka infusion protein hydrolyzed.Hiki ke hana ʻia ʻo Lysine i mea hoʻohui meaʻai me nā huaora like ʻole a me ka glucose, hiki ke maʻalahi ʻia e ka gastrointestinal tract ma hope o ka lawelawe waha.Hiki i ka Lysine ke hoʻomaikaʻi i ka hana a me ka pono o kekahi mau lāʻau.
ʻIke Huahana
Ka helu helu: 56-87-1
Maʻemaʻe: ≥98.5%
Kumu:C6H14N2O2
Kumu Wt.:146.19
Inoa Kemika: L-2,6-diaminocaproic acid;L-lysine acid base;L-hexane;L-pine
IUPAC Inoa :L-2,6-diaminocaproic acid;L-lysine acid base;L-hexane;L-pine
Lae hehee: 215°C
Solubility : He keʻokeʻo kēia huahana a kokoke paha i ka pauka crystalline kahe keʻokeʻo;ʻAneʻane ʻala ʻole.Hiki ke hoʻoheheʻe ʻia i ka wai a me ka waika formic, akā paʻakikī i ka ethanol a me ka etera.Solubility (g/100ml wai): 40 (0 ℃), 63 (20 ℃), 96 (40 ℃), 131 (60 ℃).
Nānā: He keʻokeʻo kēia huahana a kokoke i ke keʻokeʻo
Hoʻouna a mālama
E kūʻai i ka Maha: Ma kahi maloʻo, maʻemaʻe, maʻalili a me ka pahu i hoʻopaʻa ʻia.
Moku Temp
Hoʻouka a hoʻokuʻu me ka mālama, pale i ka makū a me ka lā, a mai hui pū me nā mea ʻawaʻawa a me nā mea ʻino.
Nā kuhikuhi
1. Ka hopena o ka lysine exogenous i ka hōʻike ʻana o nā genes biosynthetic mua cephamycin C a me ka hana antibiotic ma Nocardia lactamdurans MA4213.
AL Leitão et al.
Hoʻohana ʻia ka microbiology a me ka biotechnology, 56(5-6), 670-675 (2001-10-17)
I ka beta-lactam e hana ana i nā microorganisms, ʻo ka hana mua i ka biosynthesis o ke apo beta-lactam ka condensation o ʻekolu amino acid precursors: alpha-aminoadipate, L-cysteine a me D-valine.I ka Nocardia lactamdurans a me nā actinomycetes e hana ana i ka cephamycin, hana ʻia ka alpha-aminoadipate mai L-lysine e ʻelua.
2.Stryer L. a me WH Freeman
Biochemistry (3rd Edition), 19-20 (1988)
ʻO kahi loiloi spatial proteomics quantitative o ka huli ʻana o ka proteome i nā cell kanaka.
3.François-Michel Boisvert et al.
Mākaʻikaʻi molekala a me nā kelepona: MCP, 11(3), M111-M111 (2011-09-23)
ʻO ke ana ʻana i nā waiwai o nā protein endogenous cell, e like me ka pae hōʻike, subcellular localization, a me nā helu huli, ma kahi pae proteome holoʻokoʻa e noho mau ana i mea paʻakikī nui i ka wā postgenome.ʻAʻole hilinaʻi nā ʻano hana nui no ke ana ʻana i ka ʻōlelo mRNA
4.Devlin TM
Buke Haʻawina no Biochemistry: With Clinical Correlations (5th ed.), 97-97 (2002)